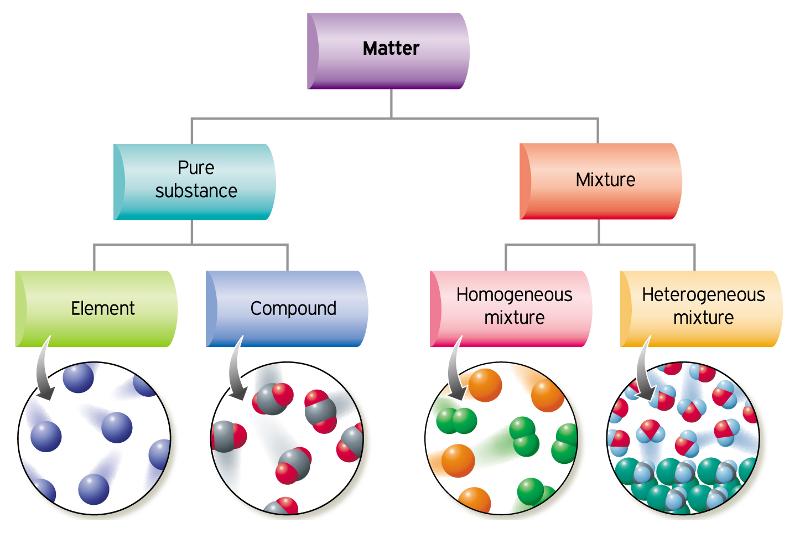
Everything around us is made of matter. Mater is anything that has mass and takes up space. We can classify matter by its physical state (solid, liquid, gas, plasma) and many other physical properties, but we can also classify it by its composition.
Pure Substances vs Mixtures:
Pure Substances
A pure substance is made of only only type of particle or matter. Sugar, distilled water and copper wire are all types of pure substances. A pure substance is the composed of the same stuff throughout which makes them appear uniform or homogeneous.
Mixtures:
A mixture, on the other hand, is made up of two or more different types of particle or mater. Sometimes it’s easy to spot the different particles, like in a chocolate chip cookie, but sometimes it’s not easy to tell that something has more than one type of particle in it. Apple juice, for example, is made of water, sugar and fruit juice, which itself is made of a number of biological molecules and water. We know this not so much because we can see the different substances, but because we can detect them with technology and our taste buds.
Mixtures can then also be divided into two categories. If the different particles are obvious and unevenly distributed, we call them mechanical mixtures or heterogeneous mixtures. If the different particles are evenly distributed throughout the mixture and it looks the same throughout, we call the mixture homogeneous or a solution.