The concept of heat can sometimes be confusing in its nature because there are some common misconceptions about its source out there. The fact that there is an absolute lowest temperature, but not really ( at least we’re not sure there is) a highest possible temperature, and the fact that the statement ” keep the cold in that cooler” is utter nonsense may be a surprise to us until we understand what heat really is.
Identifying Temperatures:
Humans use a variety of temperature scales to identify common temperatures. Internationally, the most common temperature scale is the Celsius scale, but Fahrenheit and Kelvin are also commonly used. Try to assign the following list of temperatures given in degrees Celsius to the real life situations listed:
| 15,000,000 oC | Hot tea |
| 6,000 oC | Normal human body temperature |
| 160 oC | Room Temperature |
| 100 oC | Freezing water |
| 80 oC | Interior of the Sun |
| 58 oC | Ice cream |
| 40 oC | A pizza oven |
| 37 oC | Inside of a refrigerator |
| 20 oC | Hottest air temperature ever recorded |
| 7 oC | Coldest air temperature ever recorded |
| 0 oC | Surface of the Sun |
| – 10 oC | Coldest possible temperature |
| -89 oC | Comfortably warm bath water |
| -273 oC | Boiling water |
Temperature, Heat, and the Particle Theory
Temperature and Heat are not the same thing. Heat is a form of energy that “resides’ in the movement of particles, and Temperature is the average energy of motion of those particles as measured by devices.
Particle Theory is a collection of proven realizations about particles and their motion:
1) All matter is composed of tiny particles ( we also call them molecules and atoms).
2) These particles are always moving.
3) The more energy (thermal energy or heat energy) they have, the faster and more vigorously they move.
This means that we should expect a change in motion of particle of any object that changes temperature (heating and cooling). In turn, if the particles of an object change how strongly they move, we should also expect some other changes in the object.
Expansion/Contraction:
If we take the previous thought of changes in motion of particles during heating and cooling it should not surprise us that an object changes form if we heat of cool it sufficiently. In short, an object that is heating up increases in size because its particles move more and need more room for that increased motion, and an object that is cooling down decreases (shrinks) in size because its particles move less and require less room. As a rule of thumb this applies, but not under all circumstances due to other factors that go well beyond what we need to worry about right now.
This is a picture of an expansion joint found in bridges to allow for the safe expansion and contraction of the bridge deck.
This is an image of what happens to train tracks in high temperatures if they are constructed without gaps between the track pieces.
States of Matter:
All matter exists in more than one form. We are most familiar with three (there are more) states of matter:
Solid:
In a solid particles are close together, attached by strong, short bonds and particles are held in place. They can vibrate but not change place. A solid keeps its own volume and shape.
Liquid:
Particles in a liquid are still fairly close together, but due to and increase in heat motion of its particles, a liquid does not have strong enough bonds to hold particles in a set location. Bonds are of a medium/weak nature and of a somewhat longer length. Particles can change locations but always remain connected to other particles around them. A liquid keeps its volume but takes the shape of its container. Without a container a liquid spreads.
Gas:
Particles in a gas have high energy and are virtually not connected to each other. They are very far apart from one another and are free to go wherever their momentum takes them. Gasses have a significantly increased volume compared to liquids and solids. A gas takes the shape and volume of its container.
Measuring Temperature/ Temperature Scales:
Most temperature measuring devices (thermometers) use the expansion and contraction of special substances due to changes in temperature. A simple thermometer consists of a reservoir of some liquid connected to a thin tube into which the liquid can expand when heated. For reasonable temperature ranges, the amount a liquid expands per degree temperature increase is as good as constant. The selection of the liquid depends on whether it freezes and boils outside of the desired temperature range. For example: Ethyl alcohol freezes below -100oC and boils above +78 oC. This makes it an ideal liquid for use in weather thermometers for common temperature ranges. Mercury on the other hand freezes below -38 oC, a temperature commonly experienced in a number of inhabited regions of the globe. As such, mercury is not as good a choice as alcohol. Mercury does have the advantage of being more sensitive to temperature changes and is a superior choice for medical thermometers because small differences in body temperature can indicate the presence of infection or other problems. For more information consult these entries:
http://en.wikipedia.org/wiki/Alcohol_thermometer
http://en.wikipedia.org/wiki/Mercury-in-glass_thermometer
Modern technology has yielded a new way of measuring temperature that rests on a different effect of temperature on an object. The hotter a wire becomes, the less well it conducts electricity. This change in conductivity (the ability to conduct electricity) can be calibrated and used to measure temperature accurately and quickly. Since the wire is a solid, rather than a liquid and we don’t measure expansion, the device can be small and can be used to measure extreme temperatures such as kilns and smelting ovens.
Fahrenheit:
The Fahrenheit scale is a temperature scale that is less and less used internationally with the advent of the Metric System. Being a non-metric unit, the degree Fahrenheit is to this day only used in the USA among developed nations. The increase of a degree Fahrenheit is roughly equivalent to an increase of 1/2 a degree Celsius. It is also based on the freezing point of brine ( a temperature also achieved by mixing ammonium nitrate with water) and the temperature of a healthy human. We can convert Fahrenheit into Celsius and back by the following calculations:
F to C: 1) Subtract 32 and then 2) Divide by 1.8
C to F: 1) Multiply by 1.8 and then 2) add 32
Celsius:
The Celsius scale is the most commonly used temperature scale in use today. being a metric unit it has become the common temperature scale with the exception of the USA. It is based on freezing and boiling points of pure water, zero and 100 oC respectively. It shares the size of its unit with the Kelvin Scale.
Kelvin:
The Kelvin Scale is used in scientific calculations since it is the official unit of absolute temperature. It is based on the lowest possible temperature (0 K) and as such has no negative numbers (unlike the other two temperature scales). An increase in 1K is the same as an increase of 1oC. To convert Kelvin into Celsius and back we subtract or add 273 (273.15 if we want to be exact).
K to C: subtract 273
C to K: add 273
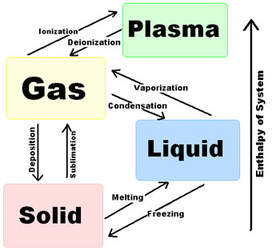
Changes of State:
The above chart shows the 4 common states of matter. We are only going to worry about the first three (solid, liquid, and gas).
Consider the arrow at the right to indicate the heat energy of the material.
Consider how different the particles of a material are arranged in the different states. A change from on arrangement to the next requires a complete re-organization of how the particles are connected to one another and how they are able to move. It shouldn’t surprise us that these changes come with an energetic cost. For example, to go from solid to liquid, a substance must break significant bonds between the particles. This uses up enormous amounts of energy. In fact, it uses up all available energy and as a consequence, a substance that is melting does not change temperature because all extra heat energy goes into the melting process eventually. The same is true for evaporation. In cooking we make use of this property by boiling our food. Overheated food scorches but if we boil our food in water, we can be guaranteed that the temperature never exceed 100 oC even if our hot plate is set to much higher temperatures.
What may be surprising and non-intuitive is that the same is true in reverse. Condensing and freezing substances never drop below their freezing/condensation point even is the exterior is significantly colder as long as there is some substance left condensing/freezing. IN nature, many animals survive the subzero temperatures of colder climates by hiding in lakes. even though the water above them is freezing solid, the liquid portion is just around 0oC. The logical conclusion from this is that a freezing liquid is releasing heat during the freezing process, keeping the liquid portion at the freezing point! This may seem strange at first but the conclusion is inescapable.
Heat and Crime Scene Investigation:
In many occasions heat can give investigators clues about a crime. A car motor produces lots of heat during its operation. If a car has recently been driven, the motor should still be warm. If it isn’t, the car has not been driven. The temperature of a human body drops at predicable rates if we know the temperature circumstances of the crime scene, allowing us to deduce, within reason, how long ago someone has died. In class you will be presented with a fictitious (means it’s not real :-)) crime that you are required to solve using your knowledge of heat. In your discussion of the crime, be sure not to over-interpret your group’s finding. If your data does not allow for a conclusive statement of guilt or innocence, say so. If it does, say so as well, but be sure to explain, using logic, why that is the case.
Heat Transfer (Conduction, Convection, Radiation):
Heat is passed from one material to another in three basic ways:
Conduction:
When particles of different materials are in direct contact with one another and, through this direct contact, exchange heat energy by bumping” into one another we call that conduction.
Convection:
When heat is passed on though the movement of particles from one area to another we call that convection. This is only possible in fluids (liquids, gasses and plasma) since particles actually have to move from one location to another, carrying heat energy with them. Particles may move on their own or are moved by an external force. Movement cause by the heat difference itself is called natural convection and movement that is driven by an external force causing an exchange of heat is called forced convection.
Radiation
Heat radiation, also called thermal radiation, is a form of electro-magnetic radiation given off by a material due to its heat. Heat radiation does not require a medium (like air or water) to travel through and even traverses complete or near vacuums like space.
Controlling Heat Transfer:
Now that we know the three ways that heat can travel we can consider how to manage such heat transfer. We constantly manage the transfer of heat in large and small ways even if we don’t think about it. Clothing, insulation of houses and lunch containers, cooking and the coating of drink containers all deal with the control of heat transfer.
Controlling heat transfer by conduction:
Not all materials conduct heat equally well. Generally speaking, the denser the particles are packed, the better heat can be transferred by conduction since particles are close to one another and “bump” into each other easily. As such, metals and stone are good heat conductors (we use metals in cooking for exactly that reason). Materials with low density such as gasses are poor heat conductors due to the fact that particles are far spaced out and do not easily “bump” into one another.
Controlling heat transfer by convection:
Convection requires the movement of fluids. The easier a fluid moves the better it can pass on heat by convection. To control convection we must control the flow of fluids. In our homes we use the flow of heated air or sometimes water to warm our houses when it’s cold. If we want to prevent convection we must prevent fluids from flowing easily. We do that by creating what are called “dead air spaces” which are compartments that are too small to allow for an effective flow of fluids. Our modern insulation bats are examples where we have trapped lots of air in between small fibres of glass that create a mesh that prevents easy flow of air but is mostly air.
Controlling heat transfer by radiation:
heat radiation passes through empty space. Even a vacuum inside a thermos is no obstacle for radiation. However, light reflecting substances often also reflect most heat radiation. Tin foil and mirroring are good strategies to avoid radiation transfer of heat. Painting a box black allows it to capture lots of heat radiation, heating up the contents.
Final Design Challenge: